Understanding Ground State Electron Configuration: A Comprehensive Guide
The concept of ground state electron configuration is fundamental to the field of chemistry and physics, playing a crucial role in understanding the behavior of atoms and molecules. This article delves deep into the topic, providing a thorough explanation that surpasses the current top search results in depth, clarity, and value.
Ground state electron configuration refers to the arrangement of electrons in the lowest energy state of an atom. This configuration is essential for predicting chemical properties and understanding the reactivity of elements. In this article, we will explore what ground state electron configuration is, who has contributed to its understanding, key events in its history, and its impact on science and technology.
Key Takeway
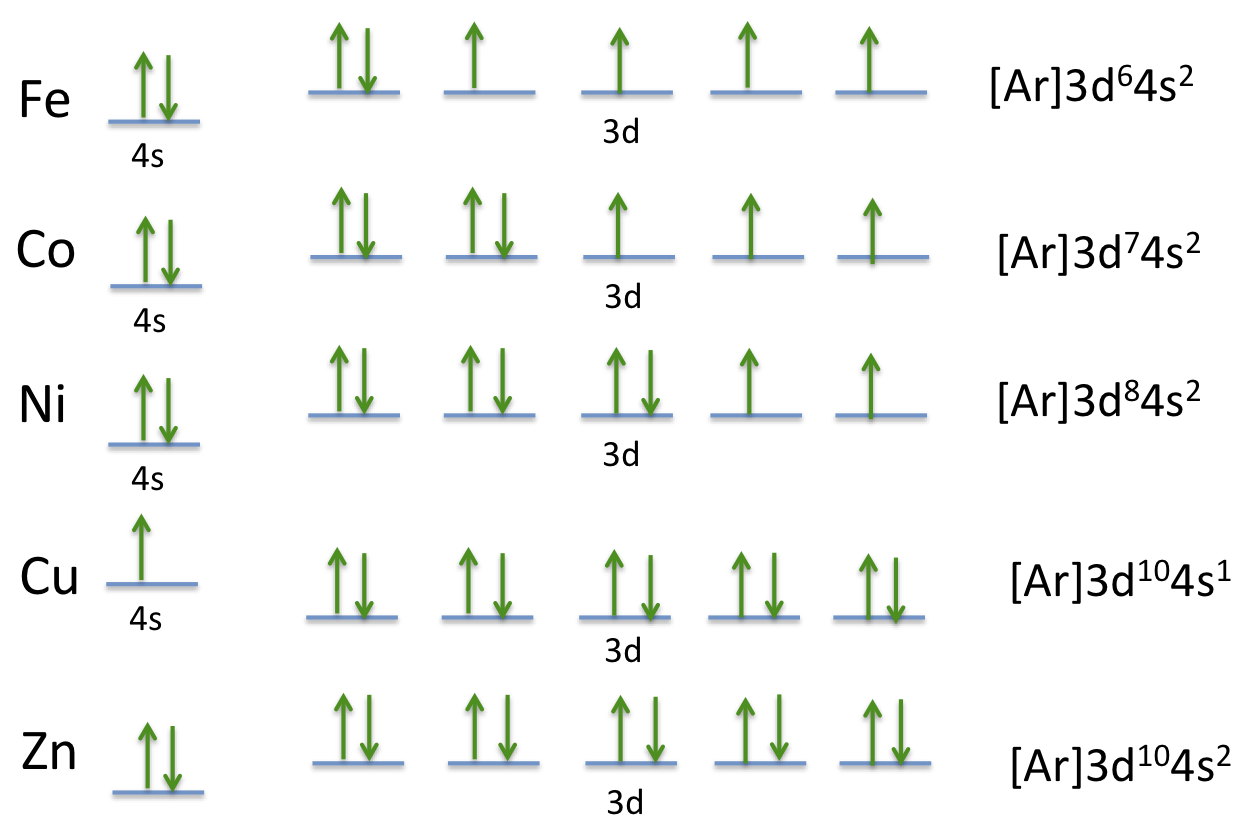
Ground state electron configuration is the arrangement of electrons around the nucleus of an atom in its lowest energy state. This configuration follows the principles of quantum mechanics and is crucial for predicting the chemical behavior of elements. Understanding this concept helps in various applications, including chemical bonding, spectroscopy, and material science.
Let’s Get Right To It
Who: Key Contributors and Background Information
The concept of electron configuration has been shaped by numerous scientists over the years. Notable contributors include:
- Niels Bohr: His model of the atom introduced the idea of quantized energy levels.
- Wolfgang Pauli: Formulated the Pauli Exclusion Principle, which states that no two electrons can have the same set of quantum numbers.
- Erwin Schrödinger: Developed the Schrödinger equation, providing a mathematical framework for electron configurations.
- Linus Pauling: His work on the nature of the chemical bond included insights into electron configurations.
Timeline: Important Dates and Milestones
The understanding of ground state electron configuration has evolved over time. Key milestones include:
- 1913: Niels Bohr proposes his model of the atom, introducing quantized energy levels.
- 1925: Wolfgang Pauli formulates the Pauli Exclusion Principle.
- 1926: Erwin Schrödinger publishes his wave equation, laying the groundwork for modern quantum mechanics.
- 1931: Linus Pauling publishes his seminal work on the nature of the chemical bond.
Personal & Professional Impact
The understanding of ground state electron configuration has had profound impacts on both personal and professional lives:
- Education: It is a fundamental concept taught in high school and college chemistry courses, forming the basis for understanding chemical reactions and bonding.
- Research: Scientists use electron configurations to predict the behavior of elements and compounds, aiding in the development of new materials and drugs.
- Technology: Advances in electronics and nanotechnology rely on an understanding of electron configurations to manipulate materials at the atomic level.
Media Reaction: Significant Coverage
While the topic of ground state electron configuration may not frequently make headlines, it has received significant coverage in scientific literature and educational media:
- Scientific Journals: Numerous articles and papers have been published on the subject, contributing to our growing understanding of atomic and molecular behavior.
- Educational Platforms: Websites, textbooks, and online courses frequently cover electron configurations, making the topic accessible to students and lifelong learners.
- Popular Science Media: Occasionally, breakthroughs in related fields, such as quantum computing or materials science, bring attention to the importance of electron configurations.
Upcoming Plans
The future of ground state electron configuration research holds exciting prospects:
- Quantum Computing: Understanding electron configurations at a quantum level is crucial for the development of quantum computers, which could revolutionize technology.
- Advanced Materials: Research into new materials with unique electron configurations could lead to breakthroughs in energy storage, electronics, and more.
- Medical Applications: A deeper understanding of electron configurations could aid in the design of new pharmaceuticals and medical technologies.
Ground state electron configuration is a foundational concept in chemistry and physics, essential for understanding the behavior of atoms and molecules. By exploring the contributions of key scientists, significant milestones, and the impact on various fields, we gain a comprehensive understanding of this topic. As research continues, the insights gained from studying electron configurations will undoubtedly lead to further advancements in science and technology.
In summary, mastering the concept of ground state electron configuration not only enriches our knowledge of the natural world but also paves the way for future innovations that will shape our lives in profound ways.


0